Трябва да сте влезли в
-
moreX
-
Компоненти
-
-
Category
-
полупроводници
- Диоди
- Тиристори
-
Електрически изолирани модули
- Електроизолирани модули VISHAY (IR).
- Електроизолирани модули INFINEON (EUPEC).
- Електрически изолирани модули на Semikron
- Електроизолирани модули POWEREX
- Електроизолирани модули IXYS
- Електроизолирани модули от POSEICO
- Електрически изолираните модули на ABB
- Електроизолационни модули от TECHSEM
- Go to the subcategory
- Мостови токоизправители
-
Транзистори
- GeneSiC транзистори
- Mitsubishi SiC MOSFET модули
- STARPOWER SiC MOSFET модули
- ABB SiC MOSFET модули
- IGBT модули от MITSUBISHI
- Транзисторни модули MITSUBISHI
- MITSUBISHI MOSFET модули
- Транзисторни модули ABB
- IGBT модули от POWEREX
- IGBT модули - от INFINEON (EUPEC)
- Полупроводникови елементи от силициев карбид
- Go to the subcategory
- Шофьори
- Силови блокове
- Go to the subcategory
- Преобразуватели за ток и напрежение LEM
-
Пасивни компоненти (кондензатори, резистори, предпазители, филтри)
- Резистори
-
Предпазители
- Миниатюрни предпазители за електронни системи серия ABC и AGC
- Бързодействащи тръбни предпазители
- Забавени вложки с GL/GG и AM характеристики
- Изключително бързи предпазители
- Британски и американски стандартни бързодействащи предпазители
- Бързодействащи предпазители европейски стандарт
- Тягови предпазители
- Предпазители за високо напрежение
- Go to the subcategory
-
Кондензатори
- Кондензатори за двигатели
- Електролитни кондензатори
- Icel филмови кондензатори
- Силови кондензатори
- Кондензатори за постояннотокови вериги
- Кондензатори за компенсация на мощността
- Кондензатори за високо напрежение
- Кондензатори за индукционно нагряване
- Импулсни кондензатори
- DC LINK кондензатори
- Кондензатори за AC/DC вериги
- Go to the subcategory
- Филтри против смущения
- Суперкондензатори
- Защита от пренапрежение
- Разкриващи емисионни филтри TEMPEST
- Go to the subcategory
-
Релета и контактори
- Теория на релетата и контакторите
- AC 3-фазни твърдотелни релета
- DC твърдотелни релета
- Регулатори, системи за управление и аксесоари
- Мек старт и реверсивни контактори
- Електромеханични релета
- Контактори
- Ротационни превключватели
-
Еднофазни AC твърдотелни релета
- Еднофазни променливотокови полупроводникови релета Серия 1 | D2425 | D2450
- Еднофазни AC полупроводникови релета CWA и CWD серия
- Еднофазни AC полупроводникови релета серии CMRA и CMRD
- Еднофазни AC твърдотелни релета PS серия
- AC твърдотелни релета двойни и четворни серии D24 D, TD24 Q, H12D48 D
- Еднофазни полупроводникови релета от серия GN
- Еднофазни променливотокови твърдотелни релета серия CKR
- Монофазни AC релета за DIN шина ERDA и ERAA СЕРИЯ
- Монофазни AC релета за ток 150А
- Двойни твърдотелни релета, интегрирани с радиатор на DIN шина
- Go to the subcategory
- AC еднофазни печатни твърдотелни релета
- Интерфейсни релета
- Go to the subcategory
- Ядра и други индуктивни компоненти
- Радиатори, Варистори, Термична защита
- Фенове
- Климатик, Аксесоари за табла, Охладители
-
Батерии, зарядни устройства, буферни захранвания и преобразуватели
- Батерии, зарядни устройства - теоретично описание
- Литиево-йонни батерии. Персонализирани батерии. Система за управление на батерията (BMS)
- Батерии
- Зарядни за батерии и аксесоари
- UPS и буферни захранвания
- Конвертори и аксесоари за фотоволтаици
- Съхранение на енергия
- Водородни горивни клетки
- Литиево-йонни клетки
- Go to the subcategory
-
Автоматизация
- Подемници Spiralift
- Части за дронове Futaba
- Крайни изключватели, Микро ключове
- Сензори, Преобразуватели
- Пирометри
- Броячи, Релета за време, Панелни измервателни уреди
- Индустриална защитна екипировка
- Светлинни и звукови сигнали
- Термовизионна камера
- LED дисплеи
- Бутони и превключватели
- Go to the subcategory
-
Кабели, Litz проводници, Тръбопроводи, Гъвкави връзки
- Проводници
- Кабелни щуцери и ръкави
- лица
-
Кабели за специални приложения
- Удължителни и компенсаторни кабели
- Кабели за термодвойки
- Свързващи кабели за PT сензори
- Многожилни кабели темп. -60°C до +1400°C
- Кабели средно напрежение SILICOUL
- Кабели за запалване
- Нагревателни кабели
- Едножилни кабели темп. -60°C до +450°C
- Железопътни проводници
- Нагревателни кабели в Ex
- Кабели за отбранителната промишленост
- Go to the subcategory
- тениски
-
Плитки
- Плоски плитки
- Кръгли плитки
- Много гъвкави плитки - плоски
- Много гъвкави плитки - кръгли
- Цилиндрични медни оплетки
- Медни цилиндрични оплетки и капаци
- Гъвкави ленти за заземяване
- Цилиндрични оплетки от поцинкована и неръждаема стомана
- Медни оплетки с PVC изолация - температура до 85 градуса
- Плоски алуминиеви оплетки
- Комплект за свързване - оплетки и тръби
- Go to the subcategory
- Тягово оборудване
- Накрайници за кабели
- Изолирани гъвкави релси
- Многослойни гъвкави шини
- Системи за управление на кабели
- Go to the subcategory
- View all categories
-
полупроводници
-
-
- Suppliers
-
Applications
- CNC машини
- DC и AC задвижвания (инвертори)
- Двигатели и трансформатори
- Енергетика
- Енергийни банки
- Заваръчни машини и заварчици
- Захранвания (UPS) и токоизправителни системи
- Измерване и регулиране на температурата
- Изследвания и лабораторни измервания
- Индукционно нагряване
- Индустриална автоматизация
- Индустриална защитна екипировка
- Компоненти за зони с опасност от експлозия (EX)
- Машини за сушене и обработка на дървесина
- Машини за термоформоване на пластмаси
- Минно дело, металургия и леярство
- Оборудване за разпределителни и контролни шкафове
- ОВК автоматизация
- Печат
- Трамвайна и железопътна тяга
-
Инсталация
-
-
Индуктори
-
-
Индукционни устройства
-
-
Обслужване
-
- Contact
- Zobacz wszystkie kategorie
Водородни горивни клетки - основна информация

The technological development and changes in the global industry force the continuous need to search for new energy sources. When it comes to batteries, the focus is not only on high efficiency, capacity, or performance but also on ecology and the ability to operate in various conditions. Therefore, the problem boils down to balancing many factors, and the solutions proposed by manufacturers focus on delivering specialized devices to the market. The electrolyte or the use of substances with properties and parameters that will influence the effectiveness of energy sources is also essential.
A breakthrough in this field has been fuel cells, which, as electrochemical devices, allow the generation of electrical energy and heat from the reactions occurring within them. In short: in fuel cells, the conversion of chemical energy from fuels such as hydrogen, methane, butane, methanol, or gasoline into electrical energy takes place.
DACPOL, addressing these needs and responding to market expectations, has introduced hydrogen-based solutions into its offer, which will be expanded in the future to include other energy sources. Currently, we offer PEM fuel cells of various power ratings, as well as complete power systems containing a ventilation system, converter, and housing.
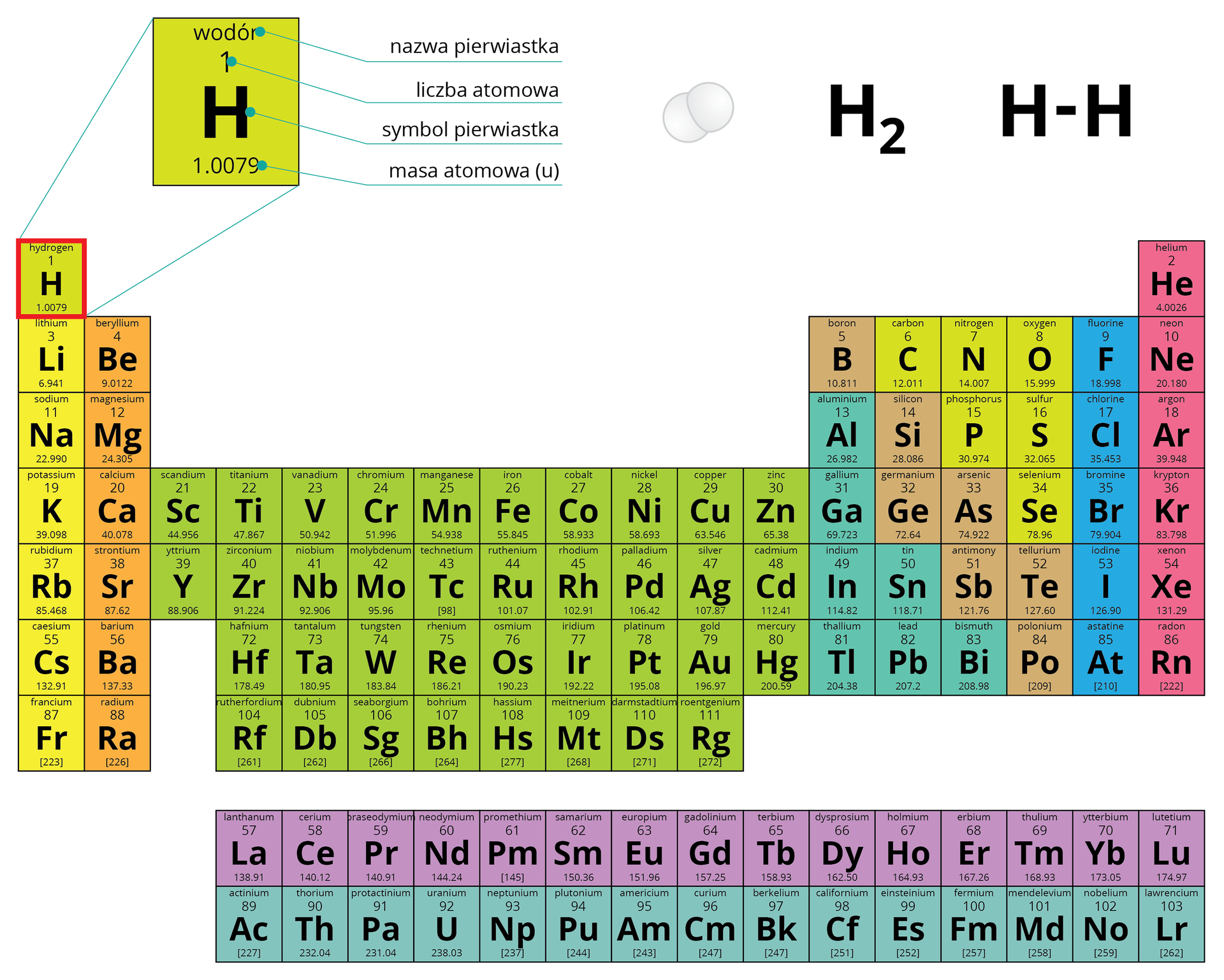
Hydrogen - The simplest and lightest element
It is the simplest (composed of one proton and one electron) and lightest (14 times lighter than air) chemical element that starts the periodic table and accounts for about three-fourths of the mass of the Universe. Hydrogen is contained in water covering about 70% of the Earth's surface, as well as in all organic matter. Under normal conditions, hydrogen is a colorless, tasteless, and odorless gas, slightly soluble in water (0.021 vol. H2 in 1 vol. H2O at 0°C). The low critical temperature of hydrogen (-239.9°C) makes its liquefaction more difficult than that of air and most other gases. Liquefied hydrogen is a colorless liquid with a specific gravity of 0.07 g/cm3. Due to its small molecular mass (2.0158), hydrogen gas exhibits the lowest specific gravity among all gases - 0.08988 g/l at 0°C and 1 atm. Compared to other gases, it also has a high diffusion speed and is the best thermal conductor among them. It is a flammable gas, burning with a clean, carbon- and soot-free flame. In comparison with other popular fuels like gasoline, methane, and propane, hydrogen has a clearly higher calorific value, heat of combustion, ignition energy, and flame speed.
Hydrogen storage
High shares of renewable energy sources in the power system can require long-term and seasonal storage, for example, to provide electricity for several days during periods of very low wind and sunlight.
Hydrogen and hydrogen fuels, such as methane, liquid organic hydrogen carriers (LOHCs), and ammonia produced by electricity through electrolysis, are potential options for long-term and large-scale energy storage. Salt caverns are the best choice for underground storage of pure hydrogen due to their tightness and low risk of contamination. Alternative underground hydrogen storage options are also being explored, such as caves, aquifers, and places left after oil and gas extraction.
Converting electrical energy into methane using energy produced from natural gas is another option for long-term hydrogen storage.
Pure hydrogen is mostly stored in pressure vessels. Few construction materials are used for manufacturing hydrogen storage tanks, as hydrogen significantly increases their brittleness. The best current solution is ultralight composite materials that withstand pressures above 20 bar. These materials are used in prototypes of cars and buses.
Among them, the following should be mentioned:
- Metal tanks made of steel, capable of withstanding 200 bar, or aluminum tanks, capable of withstanding a maximum pressure of 175 bar.
- Aluminum tanks reinforced with fiberglass, aramid, or carbon fibers, capable of withstanding maximum pressures above 250 bar.
- Bottles made of fiberglass/aramid composites or carbon fiber with a metal liner, capable of withstanding maximum pressures of 305 and 438 bar, respectively.
- Bottles made of typical carbon fiber coated with a polymer layer, capable of withstanding pressures above 661 bar.
Carbon Nanofibers - A material of the future for hydrogen storage tanks
Carbon nanofibers, including carbon nanotubes, are the material of the future for manufacturing hydrogen storage tanks. These structures have exceptional electrical and mechanical properties, resembling, under electron microscopy, a mat made from intertwined carbon threads. They conduct heat well and exhibit high strength, making them one of the strongest and stiffest materials discovered to date.
Some tanks are used for long-term hydrogen storage, while others are designed for continuous filling and emptying.
Hydrogen is also stored in liquid form. Liquid hydrogen tanks can be used in transport and portable devices. However, the technology for producing liquid hydrogen requires significant energy input, as it must be cooled to a very low temperature (-252.87°C) once compressed.
The main advantage of storing hydrogen in liquid form, rather than gas, is that it occupies a much smaller volume.
Liquefied hydrogen has found use as rocket fuel in internal combustion engines and fuel cells. During the combustion of hydrogen in a rocket engine, large amounts of energy are generated, releasing water along with trace amounts of ozone and hydrogen peroxide.
Typical hydrogen applications
- Mixtures of argon with hydrogen are used as shielding gases in plasma welding and TIG welding. These mixtures are primarily used when welding austenitic stainless steel and some nickel alloys.
- Hydrogen, combined with argon, can also be used to produce mixtures for plasma cutting (mainly for stainless steel and aluminum). In glass manufacturing, hydrogen is used to polish edges.
- Hydrogen is also used in the production of carbon steels, special metals, and semiconductors.
- In electronics, hydrogen is commonly used as a reducing agent and carrier gas.
- Other processes involving hydrogen include hydrofining of petroleum products, desulfurization of exhaust gases, heat treatment of metals, and leak testing. Hydrogen is also used in various petrochemical and chemical processes.
- Hydrogen is used to power flames in industrial and laboratory burners.
PEM Fuel Cells - How Do Hydrogen Fuel Cells Work?
The PEM (Proton Exchange Membrane or Polymer Electrolyte Membrane) technology involves supplying fuel cells with pure hydrogen or reformate. The membrane in this case is a polymer material such as Nafion. A characteristic feature of PEM fuel cells is their high efficiency in generating electricity – up to 65% – and the low amount of heat generated. Another advantage is the good responsiveness of the cell in systems subjected to varying loads, as well as the short startup time. These properties result from the low reaction temperature within the fuel cell: 60 – 100°C.

The electrolyte allows the flow of cations while preventing the flow of electrons. The chemical reaction within the fuel cell involves splitting hydrogen into protons and electrons at the anode, followed by combining the reaction substrates at the cathode. Electrochemical processes are accompanied by the flow of electrons from the anode to the cathode, bypassing the impermeable membrane. As a result of the electrochemical reaction between hydrogen and oxygen, electric current, water, and heat are produced. The fuel – hydrogen in its pure form or mixed with other gases – is continuously supplied to the anode, and the oxidizer – oxygen in its pure form or a mixture (air) – is continuously supplied to the cathode.
Fuel cells theoretically do not discharge. In practice, degradation or malfunction of components limits their lifespan. The main applications of PEM fuel cells are vehicle propulsion and the construction of stationary or portable power generators.
Advantages of Hydrogen Fuel Cells
High reliability – no moving parts, high quality of the energy supplied. The energy delivered by fuel cells is highly resistant to interference.
Hydrogen fuel cells are an ideal power source for medical devices, measuring equipment, computers, etc. Fuel cells are characterized by high efficiency and energy density.
A fuel cell is always smaller and lighter than other energy sources with comparable power.
Fuel cell efficiency in generating electricity can reach up to 50%. In the process of cogeneration, producing both electricity and heat, fuel cells achieve an efficiency of up to 85%. Various types of fuels can be used in fuel cells.
Fuel cells can be powered by any fuel rich in hydrogen. Hydrogen can be obtained from fuel either inside the fuel cell, through internal reforming, or outside the cell using an external device called a fuel reformer.
Thanks to electrolysis, hydrogen for fuel cells can be produced using alternative energy sources. Pollution resulting from the production of energy using "conventional" methods is a cause of environmental degradation and the emergence of new civilization diseases. A fuel cell produces 25 times fewer pollutants compared to internal combustion generators.
When the fuel cell is powered by hydrogen, the amount of pollutants produced is minimal. Individual fuel cells can be combined to achieve the desired power generation level (scalability). Fuel cell assemblies of different shapes are used to power everything from a single light bulb to industrial machinery. PEM fuel cell applications also include vehicle propulsion and the construction of stationary or portable power generators.
The principle of operation of the hydrogen fuel cell is well known, and progress is currently being made in the development of materials for electrodes, membranes, seals, and catalysts. The goal of research is to extend the lifespan and efficiency of the cell while reducing production costs. Additionally, new technologies for manufacturing fuel cell components are being developed by replacing mechanical processing with precise spray technologies. The effects of this research are already visible to end-users: the lifespan of fuel cells is increasing, and the cost of produced energy is decreasing.
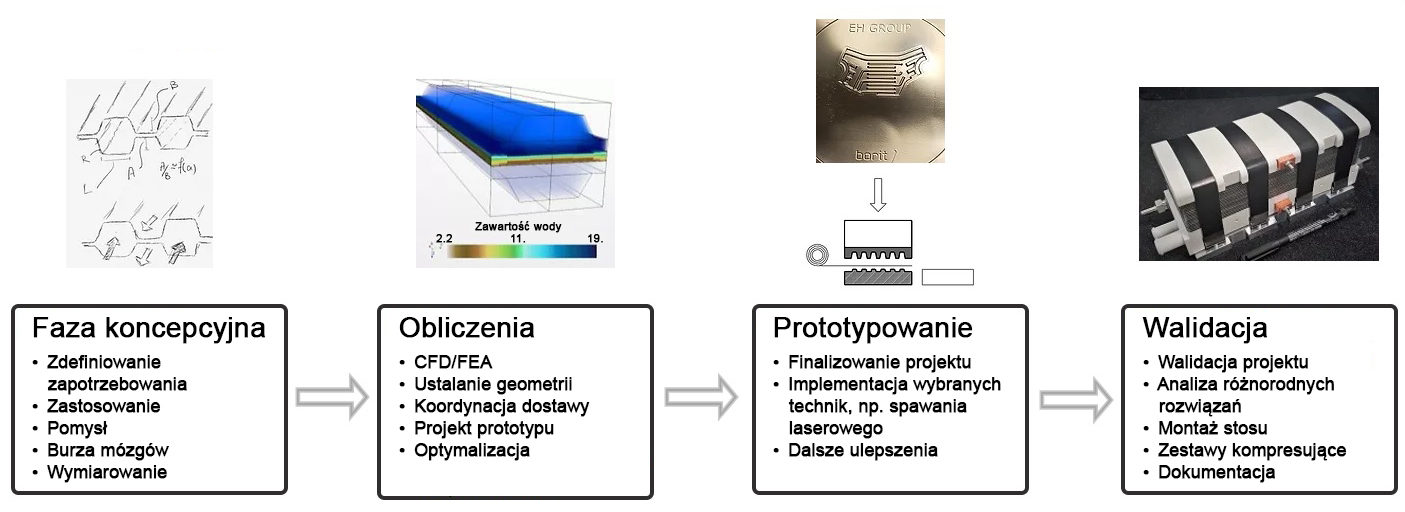
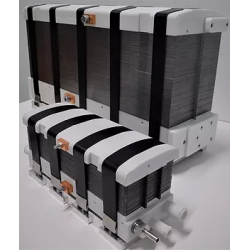
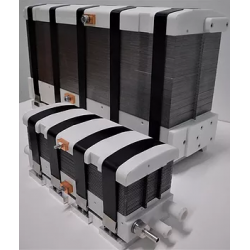
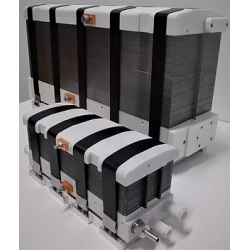
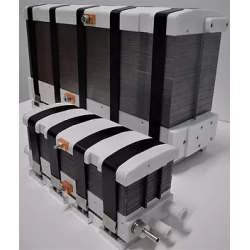
DACPOL's offer includes not only the delivery of fuel cells themselves but also complete systems and support for working with hydrogen technology.
The simplest form of cooperation is the design and construction of a hydrogen fuel cell tailored to the user's individual needs. The requirements may concern not only electrical parameters but also environmental conditions, working conditions, application, or special construction. The production phase is actually the final stage of the entire process, which begins with a thorough understanding of the customer's needs and the establishment of subsequent design steps.
Key characteristics of fuel cells supplied by DACPOL are:
- High energy density, related to the appropriately designed microstructure of the elements comprising the stack,
- Simplified construction making the stack more compact, lighter, and more efficient than similar solutions,
- Scalability enabling the construction of larger solutions (with higher power).
In addition to supplying hydrogen fuel cells as energy sources, DACPOL also offers the possibility of building a complete hydrogen-based power system for the customer. This solution includes the fuel cell, heat exchanger, inverter, and housing for mechanical protection along with assembly. We will also assist in selecting appropriate components if the user already has some of the elements of the above system.
Related products
Related posts
 Now available – DC/DC converters from PREMIUM
Now available – DC/DC converters from PREMIUM
 New release in DACPOL lighting for lathes – Kira covers
New release in DACPOL lighting for lathes – Kira covers






Leave a comment
Comments
Ogniwa wodorowe
By: Łukasz Konieczniak On 29-07-2025Dzień dobry
Mam prośbę o wycenę paliwa wodorowego22kW z linku: https://www.dacpol.eu/pl/ogniwa-paliwowe-wodorowe/product/ogniwa-paliwowe-wodorowe-eh-81-moc-22-kw
Dodatkowo prośba o podanie wytycznych jakie musze byc parametry zasilania wodorem oraz jakie parametry elektryczne uzyskujemy na wyjściu z ogniwa.
Pozdrawiam
Łukasz Konieczniak
Replied by: Michał DACPOL On 01-09-2025
Pytanie o cenę ogniwa paliwowego o mocy 5.5kW , 12 kW oraz 25kW
By: Tomasz On 15-11-2024Dobieram ogniwo paliwowe do instalacji wodorowej dla zasilania obiektu w zimę. Pozdrawiam,
Replied by: Michał DACPOL On 15-11-2024